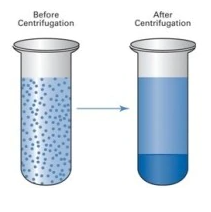
Centrifugation is a technique for separating particles from a solution based on particle size, shape, density, media viscosity, and rotor speed. Particles are suspended in liquid medium and placed in centrifuge tubes. The tube is then placed in the rotor and rotated at the specified speed. Separation by sedimentation can be done naturally under the influence of Earth’s gravity, however, it takes a long time. Centrifuges make this natural process faster.
What is pellet in centrifugation ?
Best centrifuges work on the principle of sedimentation: under the influence of gravity (g-force), substances separate according to density. Different types of separation are known, including isopycnic, ultrafiltration, density gradient, phase separation and granulation.
Typically, centrifugation applications specify the forcesof acceleration applied to the sample, rather than specifying a specific rotational speed, such as revolutions per minute. Acceleration is usually given in terms of gravity [× g] (or a multiple of xg or g force), which is the standard value of acceleration due to gravity on the Earth’s surface (9.81 m/s 2 ). The distinction between rpm and rcf is important because two rotors of different diameters running at the same rotational speed (rpm) result in different accelerations (rcf).
What factors affect centrifugation:
- Density of samples and solutions
- temperature/viscosity
- particle displacement distance
- spinning speed
There are two types of centrifuge procedures; one is preparative, which aims to separate specific particles, and the other is analytical, which involves measuring the physical properties of settled particles.
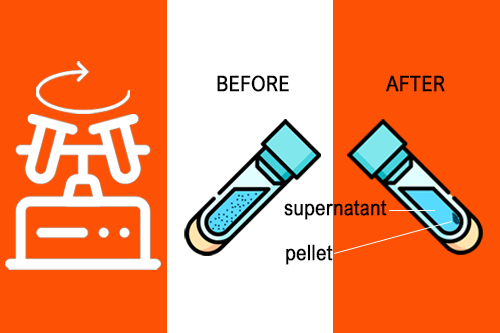
Pelletizing is the most common application of centrifuges. Here, the particles are concentrated in the form of pellets at the bottom of the centrifuge tube. and separated from the remaining solution (called the supernatant). During phase separation, chemicals are converted from a matrix or aqueous medium to a solvent (for additional chemical or molecular biology analysis). In ultrafiltration, membranes are used to purify, separate and concentrate macromolecules. Isopycnic centrifugation was performed using an “autogenous” density gradient established by equilibrium sedimentation. The method focuses on the analysis of the match with the surrounding solution. Centrifugation protocols typically specify relative centrifugal force (rcf) and acceleration in multiples g (g-force).
Pellet in centrifugation: In a solution, particles that are denser than the solvent will sink (sediment), while particles that are less dense than the solvent will float to the top.
The greater the difference in density, the faster they move. If there is no difference in density (isobaric conditions), the particles will remain stable.
Centrifuge supernatant and pellet: In a solution, particles that are denser than the solvent will sink (sediment), while particles that are less dense than the solvent will float to the top.
The greater the difference in density, the faster they move. If there is no difference in density (isobaric conditions), the particles will remain stable.
Two forces counteract the centrifugal force acting on the suspended particles
- Buoyant force: force with which the particles must displace the liquid media into which they sediment.
- Frictional force: force generated by the particles as they migrate through the solution.
Particles move away from the axis of rotation in a centrifugal field only when the centrifugal force exceeds the counteracting buoyant and frictional forces resulting in sedimentation of the particles at a constant rate.
Relative Centrifuge Force (RCF) expressed in xg (multiple of earth gravitational force)
RFC = 1,118 x R x (rpm /1000)²
R is radius in cm
rpm : Speed in Revolutions per minute
- As a result, for the same speed, a radius 20% bigger gives a number of xg 20% higher.
How to convert between multiplying gravity (×g) and centrifuge rotor speed (RPM)?
Certain procedures require precise centrifugation conditions, which must be specified in relative centrifugal force (RCF), expressed in units of gravity (multiplied by gravity or × g). Many microcentrifuges only have a speed setting (revolutions per minute, RPM) and no relative centrifugal force. Therefore, a conversion formula is required to ensure that the appropriate settings are used in the experiment. The relationship between RPM and RCF is as follows:
g = (1.118 × 10-5) x R x S²
where g is the relative centrifugal force, R is the radius of the rotor in centimeters, and S is the speed of the centrifuge in revolutions per minute. RCF values (in gravity (× g)) for common microcentrifuge rotor radii are shown in the conversion table below. For example, centrifuging a sample at 5,000 RPM in a microcentrifuge with a 7 cm radius rotor will result in a centrifugal force of 1,957 × g.
In routine sample processing procedures involving benchtop microcentrifuges, centrifugation speed and time are usually not critical factors. In general, it does not matter if the speed is faster or if the time is longer than necessary as long as the speed and time are sufficient to ensure that the cells, debris or resin are efficiently pelleted. For this reason, many protocols do not specify the specific centrifugal force to be applied, but instead indicate some general guidelines such as “centrifuge at high speed for 1 minute”.
Example of Conversion Table
| Speed (RPM) | Rotor Radius (from center of rotor to sample) in centimeters |
| 4500 | 906 | 1132 | 1358 | 1585 | 1811 | 2038 | 2264 | 2490 | 2717 | 2943 | 3170 | 3396 |
| 5000 | 1118 | 1398 | 1677 | 1957 | 2236 | 2516 | 2795 | 3075 | 3354 | 3634 | 3913 | 4193 |
| 5500 | 1353 | 1691 | 2029 | 2367 | 2706 | 3044 | 3382 | 3720 | 4058 | 4397 | 4735 | 5073 |
| 6000 | 1610 | 2012 | 2415 | 2817 | 3220 | 3622 | 4025 | 4427 | 4830 | 5232 | 5635 | 6037 |
| 6500 | 1889 | 2362 | 2834 | 3306 | 3779 | 4251 | 4724 | 5196 | 5668 | 6141 | 6613 | 7085 |
| 7000 | 2191 | 2739 | 3287 | 3835 | 4383 | 4930 | 5478 | 6026 | 6574 | 7122 | 7669 | 8217 |
| 7500 | 2516 | 3144 | 3773 | 4402 | 5031 | 5660 | 6289 | 6918 | 7547 | 8175 | 8804 | 9433 |
| 8000 | 2862 | 3578 | 4293 | 5009 | 5724 | 6440 | 7155 | 7871 | 8586 | 9302 | 10017 | 10733 |
| 8500 | 3231 | 4039 | 4847 | 5654 | 6462 | 7270 | 8078 | 8885 | 9693 | 10501 | 11309 | 12116 |
| 9000 | 3622 | 4528 | 5433 | 6339 | 7245 | 8150 | 9056 | 9961 | 10867 | 11773 | 12678 | 13584 |
| 9500 | 4036 | 5045 | 6054 | 7063 | 8072 | 9081 | 10090 | 11099 | 12108 | 13117 | 14126 | 15135 |
| 10000 | 4472 | 5590 | 6708 | 7826 | 8944 | 10062 | 11180 | 12298 | 13416 | 14534 | 15652 | 16770 |
Separation is a critical step in your workflow; therefore, it is important to consider the centrifuge requirements and technical specifications of your application. Thereby choosing the right speed and gravity to explore the latest trends in centrifuges.
PRP Centrifuge
Safe and easy-to-use centrifuges combine power with versatility and convenience. The PRP centrifuge can support all your microvolume protocols – including nucleic acid minipreps, spin columns, PCR tubes and tubes, and hematocrit capillaries – in a small footprint.
Benchtop Mini Centrifuge
Primarily used for large quantities of rapidly precipitating materials such as yeast cells, red blood cells, etc., they provide maximum capacity in a compact footprint. Adapt to a multi-lab environment with the flexibility to adapt to your changing research and clinical needs:
- Clinical application: clinical chemistry, clinical microbiology, hematology, immunology, clinical research.
- Research Applications: Cell Biology, Microbiology, Genomics/Molecular Biology, Proteomics, Biochemistry, Pharmaceutical Research.
Large Volume Centrifuge
Large volume centrifuges designed for daily work laboratories. Compatible with large and small volume sample processing. .Provides reproducible separations for high-throughput applications such as blood banking and bioprocessing. By choosing from different types of horizontal, angle rotors or even custom rotors, a range of tube capacities and shapes can be flexibly used.
96 Well Plate Centrifuge
96 well Plate Centrifuge is designed for quick spins of samples in PCR plates. Primarily designed for use with 96 well plates and small volumes, it can accommodate skirted, non skirted and semi skirted styles.
Medical Centrifuge
A medical centrifuge is a medical device used in a hospital medical laboratory. Mainly used for blood separation, DNA research, and many other routine hospital applications. Serum and blood cells are separated, and the sera are used in various biochemical assays. Therefore, the medical device centrifuge is an essential and important equipment in the hospital laboratory.
Gel Card Centrifuge
A gel card centrifuge is also called “blood type card centrifuge” or “blood group centrifuge”, because it is specially designed with rotors to suit gel microcolumn blood type cards. It is used in blood serology, blood type identification tests before crossmatching, erythrocyte washing, microcolumn agglutination, immunology, and other tests.
Centrifuge rotors are divided into three categories: pendulum rotors, fixed angle rotors and vertical rotors. Each category aims to address three key factors:
1) type of centrifugation (differential, zone velocity or isodensity),
2) speed,
3) Volume range.
Of these categories, fixed angle and swinging bucket rotors are the most common types used in benchtop, low-speed, and high-speed floor-standing centrifuge applications. Vertical rotors are mainly used for ultracentrifugation.
Fixed-angle rotor
The obvious advantage is that there are no moving parts in the rotor. This results in lower metal stress (longer service life), higher maximum g-forces are possible, and for many applications, faster centrifugation times can be achieved. The limited capacity (less flexibility) of fixed angle rotors is the only downside. The position of the particle is largely determined by the angle of the tube, which lies from the side to the bottom of the tube as it rotates. The tubes of most rotors have a 45° angle. The larger the angle of the tube, the tighter the pellet. A smaller rotor angle results in a more dispersed particle area.
Swing-bucket rotor
This rotor is very flexible and can use different tube formats, including SBS format plates, based on an extensive adapter system and high sample capacity. The moving swinging bucket parts cause increased metal stress on the rotor and bucket. Because the bucket weight puts the load on both pivots and grooves. Therefore, the maximum g-force of the swinging bucket rotor is limited to a lower level compared to fixed angle rotors, resulting in longer centrifugation times. Based on the swinging bucket principle, the particles are located at the bottom of the tube (the horizontal position of the tube during the run). Compared with the pellets located on the tube side, it is easier for the user to recycle.
Vertical Tube Rotor
Vertical rotors are quite specialized – they are most commonly used in ultracentrifugation for isopycnic separations, especially bands of DNA in cesium chloride. In this type of separation, the density range of the solution contains the same density as the particles of interest; therefore the particles will be oriented within this part of the gradient. Isodensity separation does not depend on the path length of the gradient, but on the runtime, which must be sufficient to orient the particles in the proper position within the gradient.
The K-factor of a vertical rotor is very low (usually in the range of 5-25), which means that the particle can only travel a short distance to reach the particle (or form a band in this case); thus the run time is minimized. Once it is determined that a vertical rotor is suitable for the end user application, volume and speed become the determining factors for which rotor to use.